2D Conjugated Polymers
Dr. Mingchao Wang
Linear π-conjugated polymers have attracted great interest in (opto)electronic devices. Their charge conduction occurs effectively along the polymer chain, while the interchain charge transfer sets a bottleneck via inefficient hopping. The extension of p-conjugation can, in principle, provide multiple strands for charge transport in conjugated polymers, which may significantly enhance the electronic performance.
Research Area I: 2D polymerization methodology
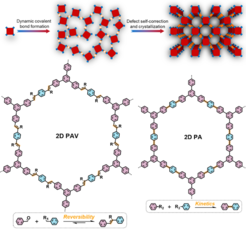
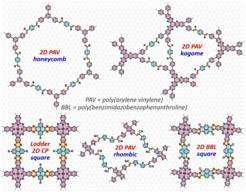
Crystalline two-dimensional (2D) poly(arylene vinylene)s (2D PAVs, also referred to as vinylene- or sp2-carbon (C=C)-linked 2D π-conjugated covalent organic frameworks) exhibit π-conjugation far beyond that of 2D polyimines. However, the solvothermal synthesis of highly crystalline 2D PAVs with large domain sizes remains challenging due to the relatively low reversibility of the C=C bond formation. In this regard, we like to gain deeper understanding of the reaction kinetics of the 2D polymerization methodologies developed so far (i.e., Knoevenagel, Aldol-type, Horner-Wadsworth-Emmons or Wittig 2D polycondensation), and to facilitate the reaction reversibility under controlled conditions, towards achieving highly crystalline 2D PAVs. We will also establish rational synthetic methodologies to develop novel 2D conjugated polymers such as 2D polyarylenes (PAs), ladder-type 2D conjugated polymers, etc.
Research Area II: Unprecedented 2D conjugated polymers
2D conjugated polymers are emerging semiconductor candidates for organic electronics. We aim to tailor their topologies, electronic structures, and functionalities by engineering the polymer backbones with various photo-/electro-/spin-/redox active building blocks, and discover nontrivial physicochemical properties.
Thiophene-based 2D conjugated polymers
Polythiophene and its derivatives have attracted considerable attention due to their easy synthesis, strong π-conjugation and tunable electronic structures. We consider that the incorporation of highly planar, polarizable, and electron-rich thiophene units into 2D conjugated polymers (e.g., 2D PAVs, 2D PAs) would enhance the charge transport performance and potentially lead to unique electronic structures. We are interested in the design and synthesis of thiophene-based donor-donor and donor-acceptor-type 2D conjugated polymers.
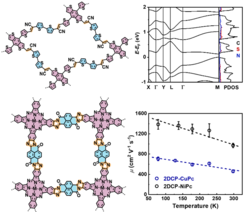
Ladder-type 2D conjugated polymers
To increase effective π-conjugation length (compared with the single-double bond alternation in polyimines and PAVs), ladder-type conjugated polymers, such as graphene nanoribbons and poly(benzimidazobenzophenanthroline) with two or more independent but tied strands of bonds, represent a unique molecular geometry to strengthen the parallel p-orbital interactions. We aim at the development of extensively p-delocalized ladder-type 2D conjugated polymers, e.g., 2D poly(benzimidazobenzophenanthroline) (2D BBL).
Research Area III: Integration and functional devices

The emerging 2D conjugated polymers featuring semiconductor properties, high mobility, and tailorable bandgaps have been expected to be outstanding substitutes for (opto-)electronics, e.g., organic field-effect transistors, organic solar cells, organic light-emitting diodes, organic photodetectors, sensors. Rationally designed 2D conjugated polymers can additionally enable the interaction between charge carriers and localized spins, hence providing promise of magnetic ordering. We are interested in the fundamental understanding of the optical, electronic and magnetic properties of 2D conjugated polymers, thus establishing unambiguous structure-property relationships. We will target at the top-down exfoliation approaches to synthesize few-layer, solution-processible 2D conjugated polymers, and the incorporation of these materials into functional devices. Beside the above characteristics, these materials also process unique features such as molecularly defined active sites, tunable porosity, redox activity, etc. thereby enabling them for multifunctional electrode materials in electrochemical energy storage (e.g., batteries, supercapacitors, etc.) and catalysis.



