Ion-selective 2D polymer membrane works as the battery electrode skin
New Publication in Nature Communication
The fast-kinetics, high-potential, and large-capacity anion-intercalation chemistries of graphite have the potential to construct sustainable batteries with promising energy and power breakthroughs. Yet, the poor durability hinders their deployment in practical batteries due to the detrimental electrolyte decomposition and cation/solvent co-intercalation.
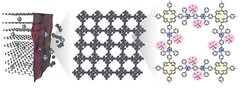
The scientists from TU Dresden and MPI Halle, together with collaborators, report the use of an ultrathin, positively charged two-dimensional poly(pyridinium salt) membrane (denoted C2DP) as the graphite electrode skin to overcome the critical durability problem. The large-area C2DP membrane (28 cm2) enables the conformal coating on the graphite electrode, alleviating the electrolyte decomposition and the formation of the anion-blocking passivation layer. The dense face-on oriented single crystals with the ultrathin thickness (20 nm) and cationic backbones allow C2DP with high anion-transport capability (PF6− diffusivity: ~10−7 cm2 s−1) and selectivity (PF6− transference number of the polypropylene separator vs. the C2DP-loaded polypropylene separator, 0.17 vs. 0.51). Such desirable anion-transport properties of C2DP prevent the cation/solvent co-intercalation into the graphite electrode and suppress the consequent structure collapse. An impressive PF6−-intercalation durability is demonstrated for the C2DP-covered graphite electrode, with capacity retention of 92.8 % after 1,000 cycles at 1 C and Coulombic efficiencies of > 99%, which is the best among the reported anion-intercalation chemistries of graphite in organic electrolytes. The study showcases the feasibility of constructing artificial ion-regulating electrode skins with precisely customized two-dimensional polymers, which offers viable means to promote problematic battery chemistries that rely on complex charge carrier ions.
Acknowledgements: this work was financially supported by European Union’s Horizon 2020 research and innovation programme (GrapheneCore3 881603, LIGHT-CAP 101017821), M-ERA.NET and Sächsisches Staatsministerium für Wissenschaft und Kunst (HYSUCAP 100478697 & Sonderzuweisung zur Unterstützung profilbestimmender Struktureinheiten), and German Research Foundation (DFG) within the Cluster of Excellence, CRC 1415 (Grant No. 417590517), and Polymer-based Batteries (SPP 2248, RACOF-MMIS). P.B. acknowledges the Alexander-von-Humboldt foundation for funding. R.D. appreciates the funding support from ERC starting grant (FC2DMOF, No. 852909). C.N. and A.T. acknowledge financial support of the DFG through a research infrastructure grant INST 275/357-1 FUGG and the Thüringer Ministerium für Wirtschaft, Wissenschaft und Digitale Gesellschaft (TMWWDG) and the Thüringer Aufbau Bank (TAB) within the project LiNaKon (2018 FGR 0092). The authors acknowledge the use of the facilities in the Dresden Center for Nanoanalysis (DCN) at the Technische Universität Dresden, the GWK support for providing computing time through the Center for Information Services and High-Performance Computing (ZIH) at TU Dresden, beam time allocation at beamline P02.1 at the PETRA III synchrotron (DESY, Hamburg, Germany), beam time allocation at beamline ID13 of the European Synchrotron Radiation Facility (ESRF), and the Helmholtz-Zentrum Berlin für Materialien und Energie for the allocation of synchrotron radiation beamtime (beamline U41-TXM).












