2D Materials Exfoliation & Printable Devices
Dr. Ali Shaygan Nia
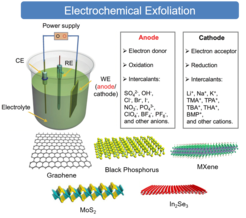
To assess high-quality 2D materials from the bulky crystals, the delamination under external stimulus control is crucial. Electro-chemical exfoliation of layered materials offers high yield, excellent efficiency, simple instrumentation, and up‐scalability, therefore it represents a key technology to advancing fundamental studies and industrial applications. Moreover, the solution processability of functionalized 2D materials enables the fabrication of opto-electronic and energy devices via different printing technologies such as inkjet and 3D printing. In our department, we explore the controlled electrochemical exfoliation process by intercalation of ions/molecules within layered crystals, fundamental physical/chemical properties as well as applications of emerging 2D materials such as black phosphorous, MXene, semi metals and transition metal dichalcogenides. We also aim to establish robust electro-chemical exfoliation methodologies for the in-situ functionalization, enabling the enhanced functionality of 2D materials and their processability via printing technologies.
