Synthetic Carbon Nanostructures
Dr. Ji Ma
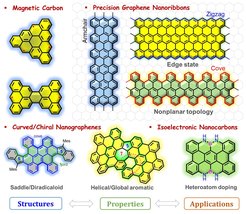
Magnetic Carbons (Kekulé vs Non-Kekulé)

Intense research efforts have been dedicated to inducing magnetism in carbon-based nanomaterials owing to their improved magnetic performance, such as lower spin-orbit couplings and larger spin correlation lengths, than many inorganic materials. Non-Kekulé polycyclic hydrocarbons (PHs) (or nanographenes (NGs)) belong to the class of carbon-rich molecules for which no Kekulé valence structures can be drawn without leaving unpaired electrons, which makes such compounds inherently magnetic and attractive for exploring their fundamental physicochemical properties. Here we are interested in the synthesis of novel non-Kekulé molecules and open-shell Kekulé PHs as well as their expanded 0D spin chain and 2D spin lattice, which exhibit unique electronic and magnetic properties as well as the potential applications for organic electronics and spintronics.
Precision Graphene Nanoribbons

Graphene nanoribbons (GNRs) are considered to be next-generation carbon materials for the fabrication of nanoscale electronic devices owing to their high electrical conductivity and tunable bandgaps. We are particularly interested in synthesizing structurally well-defined GNRs with different topologies through surface-assisted or solution-mediated approaches. Programming the shape, width, length, and edge structures of GNRs by the bottom-up organic synthetic methods, allows us to achieve unique insights into their structure-property relationships. Solution processability of is an important concern for us to readily transfer them on the substrate for reliable device fabrications, hence, synthetic control of GNRs with enhanced solution dispersibility GNRs by either introducing curvature/helicity in the ribbon backbone or installing bulky substituents on the ribbon periphery is also our consideration.
Curved/Chiral Nanographenes
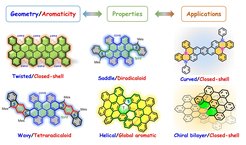
Curved/chiral nanographenes (NGs) have grown to become an important field of research due to their fascinating intermolecular packing, extraordinary chiroptical properties and dynamic behavior resulting from their contorted conformation, which are not shared by the traditional “flatland”. To date, two distinct strategies have been established for the synthesis of curved NGs, including the imposition of non-hexagonal rings and the introduction of steric strain in the molecular skeleton. The increased solubility, novel aromaticity, helicity, chirality, and curvature-dependent opto-electronic properties are key features of this class of nanocarbons. We are particularly interested in controlling the geometry, the ground state and the aromaticity of curved π-conjugated systems with the aim of the development of novel functional materials for organic optoelectronics, chirality-induced spin selectivity (CISS) devices and quantum computing.
Isoelectronic Carbon Nanostructures
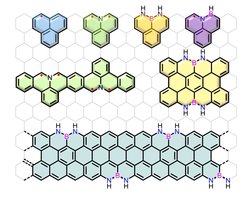
Replacing single or multiple carbon atoms in the sp2-carbon framework by heteroatoms such as nitrogen, boron, oxygen, sulfur, phosphorous or a combination of them, is an efficient and unique pathway to tune their optoelectronic properties without changing the structural skeleton. Additionally, the stability and aromaticity of the resulting carbon nanostructures can be strongly influenced. We are particularly interested in developing new methodologies to synthesize the isoelectronic carbon nanostructures and explore their new chemistry and functions for electronics and energy-related fields. The fundamental aspects of chemistry and physics of the heteroatom-doped carbon nanostructures are the core of our interests.




